Hit invasive candidiasis hard and early with high plasma drug concentrations1
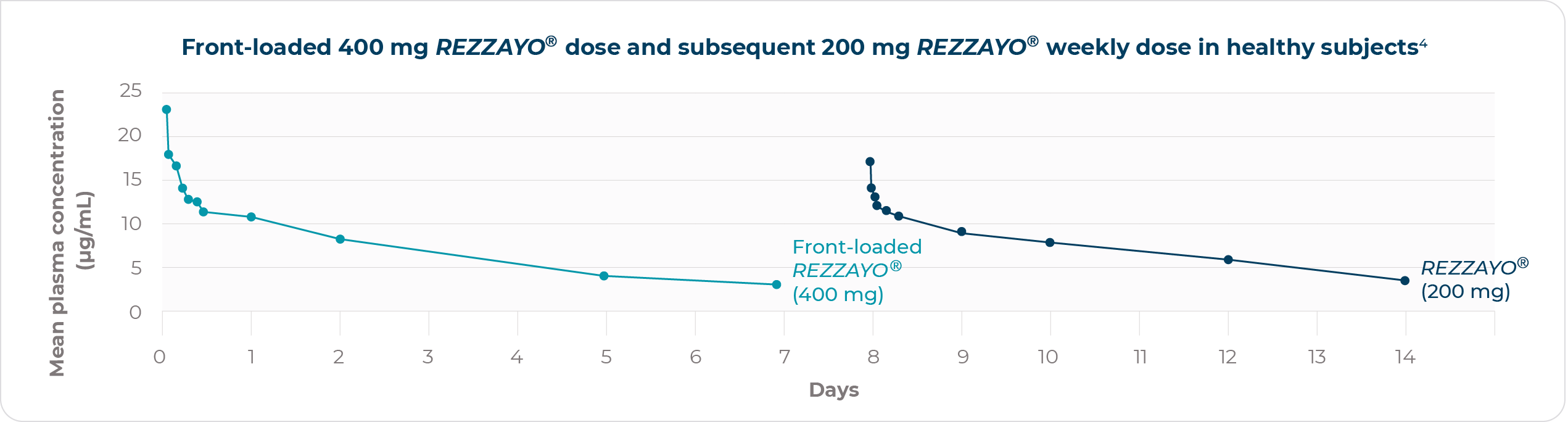
Early, lasting high plasma drug concentrations4

REZZAYO® (rezafungin for injection) is an echinocandin antifungal indicated in patients 18 years of age or older who have limited or no alternative options for the treatment of candidemia and invasive candidiasis. Approval of this indication is based on limited clinical safety and efficacy data.
Limitations of Use
REZZAYO® has not been studied in patients with endocarditis, osteomyelitis, and meningitis due to Candida.
Contraindications
REZZAYO® is contraindicated in patients with known hypersensitivity to rezafungin or other echinocandins.
Warnings and Precautions
Adverse Reactions
Most common adverse reactions (incidence ≥ 5%) are hypokalemia, pyrexia, diarrhea, anemia, vomiting, nausea, hypomagnesemia, abdominal pain, constipation, and hypophosphatemia.
Please see full Prescribing Information for REZZAYO® (rezafungin for injection).
References:1. REZZAYO®. Prescribing information. Melinta Therapeutics, LLC; 2023. 2. Thompson GR 3rd, Soriano A, Cornely OA, et al. Rezafungin versus caspofungin for treatment of candidaemia and invasive candidiasis (ReSTORE): a multicentre, double-blind, double-dummy, randomised phase 3 trial. Lancet. 2023;401(10370):49-59. doi:10.1016/S0140-6736(22)02324-8 3. Thompson GR, Soriano A, Skoutelis A, et al. Rezafungin versus caspofungin in a phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis: the STRIVE trial. Clin Infect Dis. 2021;73(11):e3647-e3655. doi:10.1093/cid/ciaa1380 4. Melinta Therapeutics. Data on file. 2023.