Enable echinocandin continuity of treatment
Simplify your patients’ treatment
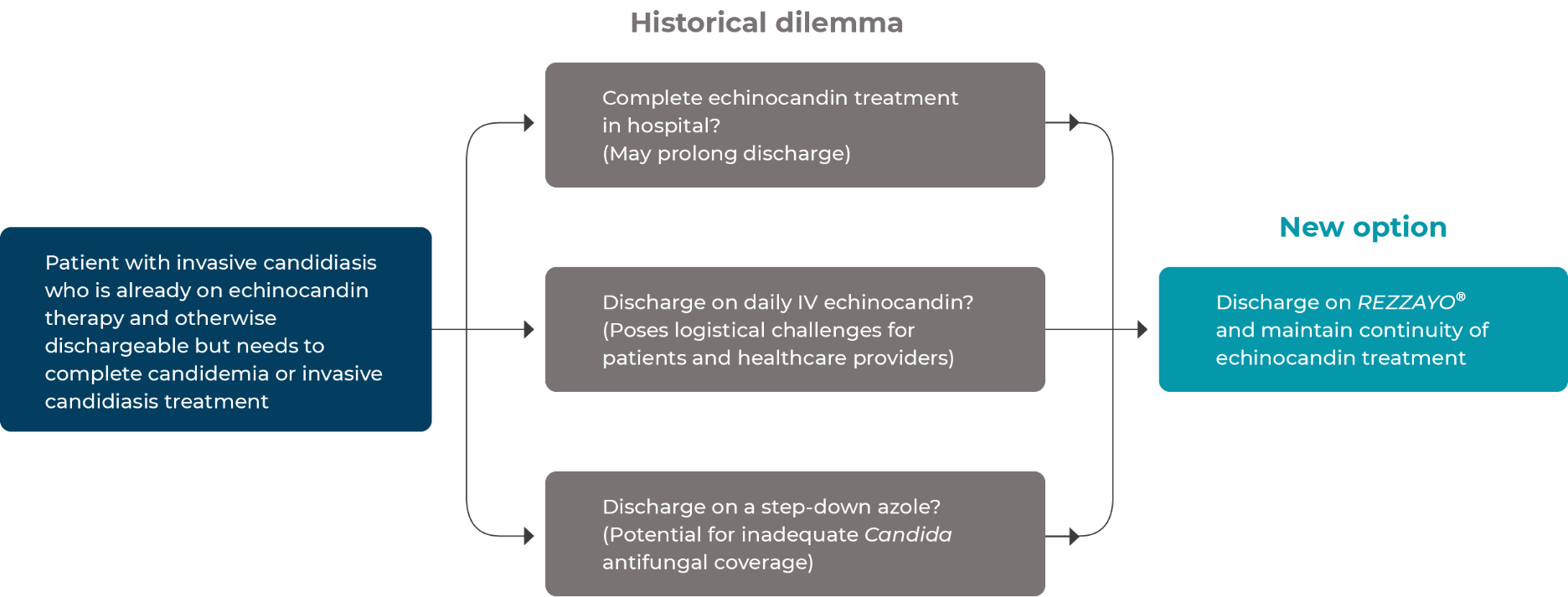
Daily intravenous administration of currently approved echinocandins for invasive candidiasis may delay discharge or limit the continuity of echinocandin therapy through discharge.1
A once-weekly IV infusion of REZZAYO® could2:
Allow for earlier discharge of patients on echinocandins who are otherwise dischargeable3
Eliminate the need for dose modification of concomitant medications due to drug-drug interactions2
Help minimize the potential for inadequate Candida antifungal coverage for patients with azole-resistant isolates or isolates of unknown susceptibility4
Limitations of Use
REZZAYO® has not been studied in patients with endocarditis, osteomyelitis, and meningitis due to Candida.
![]()
When patients are ready, you can set them on the path to echinocandin continuity of treatment with REZZAYO®
REZZAYO® offers a new option for continuity of echinocandin treatment in the outpatient setting
Potential benefits of treatment with
once-weekly REZZAYO®:
May be more convenient and manageable for patients to schedule weekly rather than daily infusions1,5
May be logistically easier for outpatient infusion vs daily infusions1,5
Allows providers to maintain patients on recommended echinocandin therapy until treatment is complete1,5
No need for a PICC line and associated maintenance, risk of complications, and discomfort from the device1,5
No known clinically significant drug-drug interactions, and no dose adjustment is needed for any known factors2
May provide greater transparency into adherence with 7 days of therapy in every dose2
PICC=peripherally inserted central catheter.
 US
US BR
BR DE
DE ES
ES UK
UK
