Choose proven echinocandin efficacy now in a once-weekly formulation1,2
REZZAYO® was evaluated for treatment of adult patients with invasive candidiasis and candidemia in the phase 3 ReSTORE trial and the supportive phase 2 STRIVE trial1-3
ReSTORE: pivotal phase 3 study of REZZAYO®1,3
ReSTORE was a prospective, double-blind, randomized noninferiority phase 3 study of once-weekly intravenous REZZAYO® vs daily caspofungin for the treatment of candidemia and invasive candidiasis in patients age 18 and older.1,3
Study design1,3
mITT N=187
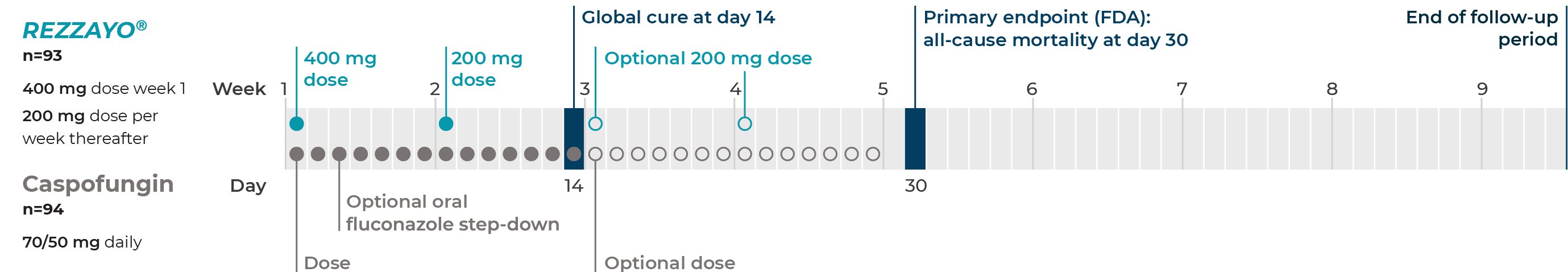
Number of patients shown in each arm is the mITT population, not the assigned and randomized numbers.
Patients in the REZZAYO® group received IV placebo on other study days to maintain masking.3
In the caspofungin group, optional oral fluconazole step-down therapy was permitted after ≥3 days of IV therapy if the patient met the criteria for cure and was preparing for discharge. Patients in the REZZAYO® group who were switched to step-down therapy continued to receive intravenous REZZAYO® once a week and daily oral placebo to maintain the masking.1,3
FDA=Food and Drug Administration; mITT=modified intent-to-treat.
ReSTORE endpoints1,3
Two primary efficacy endpoints:
- 30-day all-cause mortality (FDA)
- Global cure* at day 14
*Global cure defined as3:
For patients with invasive candidiasis documented by radiological or imaging evidence at baseline: clinical cure as assessed by the investigator, radiological cure, and mycological eradication, as confirmed for all 3 by an independent, blinded, data-review committee.
For patients with positive blood culture at screening: mycological eradication was determined by a negative blood culture after the first dose of study drug with no subsequent positive culture.
For patients with positive culture from normally sterile site other than blood: mycological eradication was either documented (as determined by a negative culture on the day of assessment [eg, day 5 or day 14]) or presumed (as determined by clinical and radiological cure [for those with evidence of disease on imaging at baseline] if a specimen from the infected site was not available).
Patient characteristics in the intent-to-treat population3
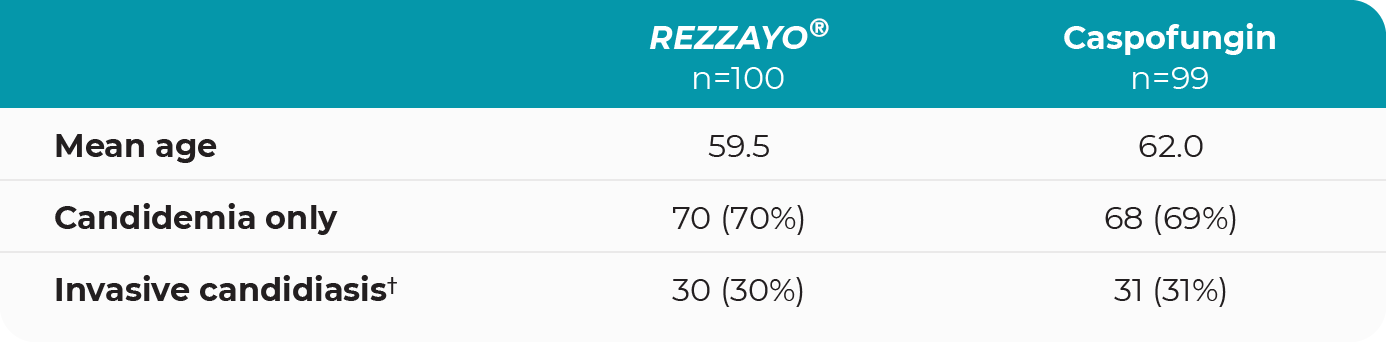
†Includes patients who progressed from candidemia to invasive candidiasis based on radiological or tissue or fluid culture assessment up to day 14.3
Isolated Candida species were similar across treatment groups3
Distribution was consistent with reported US rates, with C. albicans the most frequently isolated species followed by C. glabrata.3-5

‡Modified intent-to-treat population.
§One isolate was confirmed only by the local laboratory.3
ReSTORE: pivotal phase 3 study of REZZAYO®3
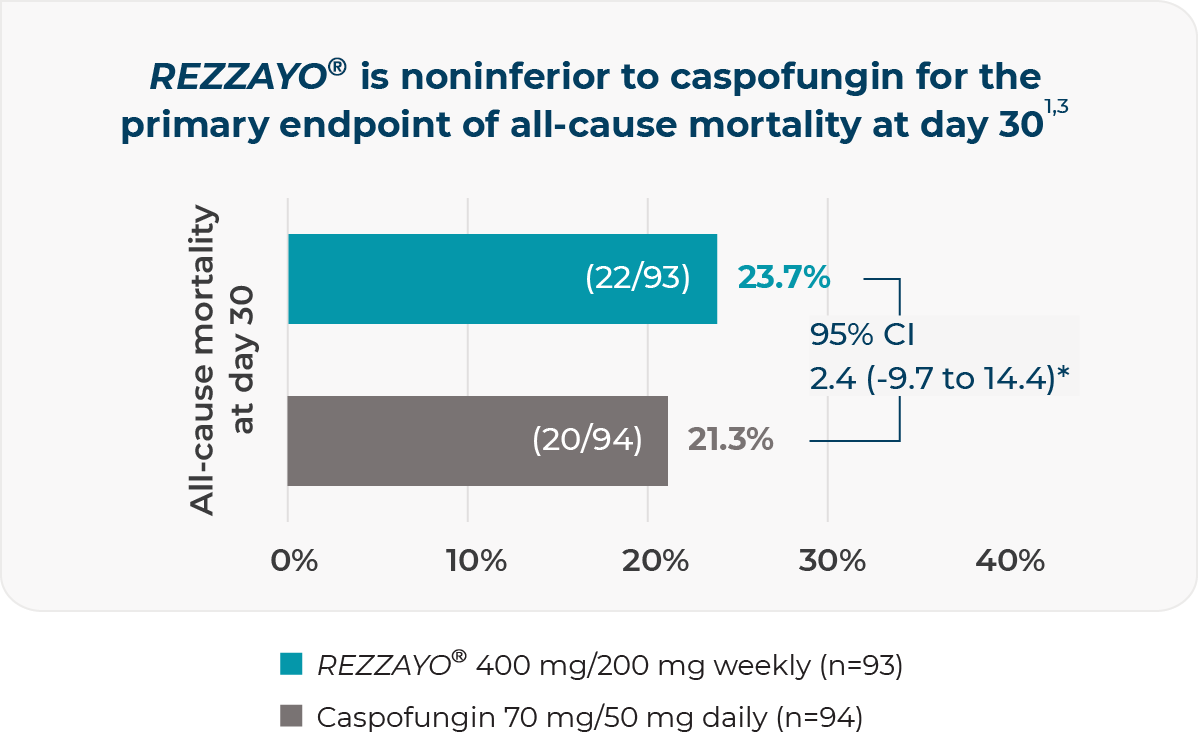
PRIMARY ENDPOINT: all-cause mortality at day 30 (FDA)3
Noninferiority was to be concluded if the upper bound of the 95% CI was lower than 20%3
CI=confidence interval.
*Two-sided 95% CI for the observed difference (%), REZZAYO® group minus caspofungin group.
![]()
Once-weekly IV infusion of REZZAYO® was noninferior to daily IV infusions of caspofungin in the modified intent-to-treat population1,3
ReSTORE: pivotal phase 3 study of REZZAYO®3
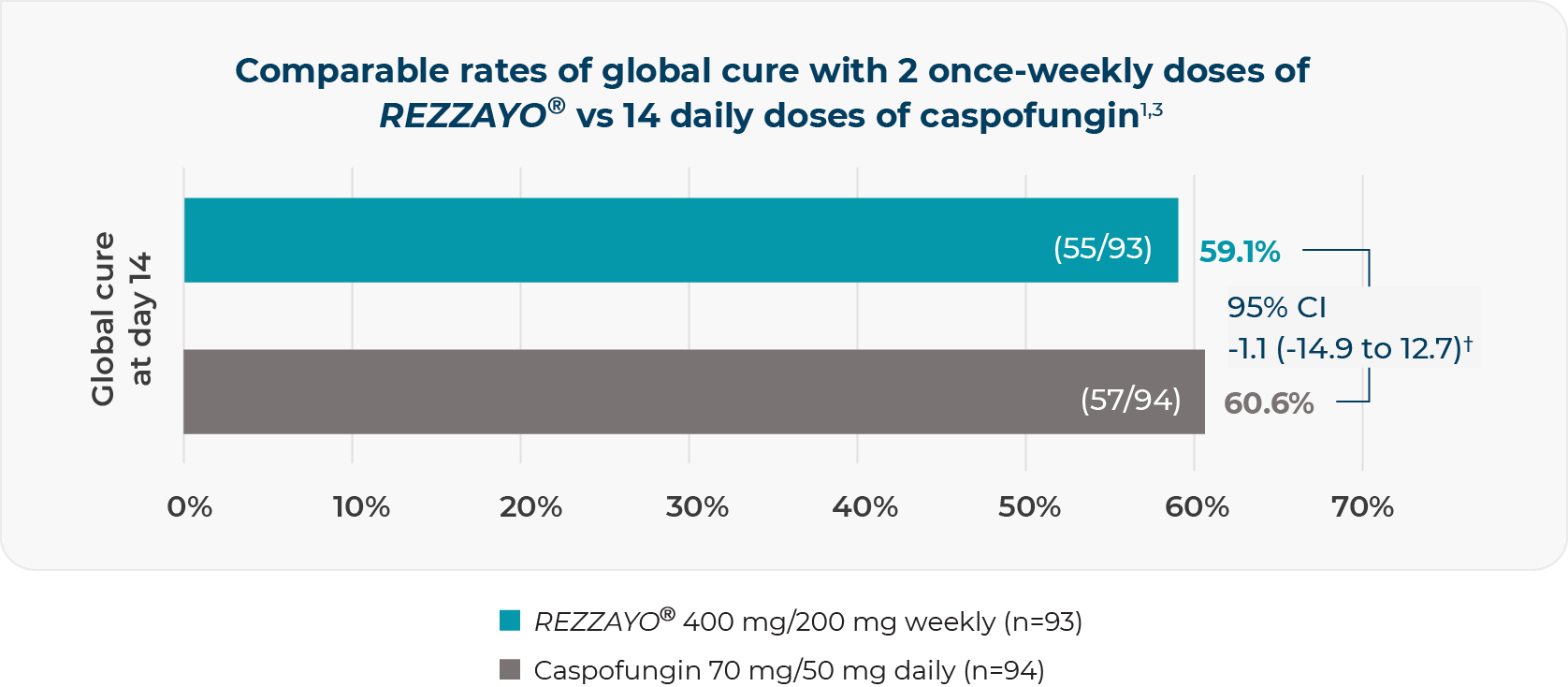
PRIMARY ENDPOINT: global cure* at day 143
Noninferiority was to be concluded if the upper bound of the 95% CI was lower than 20%3
CI=confidence interval.
*Global cure defined as3:
For patients with invasive candidiasis documented by radiological or imaging evidence at baseline: clinical cure as assessed by the investigator, radiological cure, and mycological eradication, as confirmed for all 3 by an independent, blinded, data-review committee.
For patients with positive blood culture at screening: mycological eradication was determined by a negative blood culture after the first dose of study drug with no subsequent positive culture.
For patients with positive culture from normally sterile site other than blood: mycological eradication was either documented (as determined by a negative culture on the day of assessment [eg, day 5 or day 14]) or presumed (as determined by clinical and radiological cure [for those with evidence of disease on imaging at baseline] if a specimen from the infected site was not available).
†Two-sided 95% CI for the observed difference (%), REZZAYO® group minus caspofungin group.
STRIVE: supportive phase 2 study of REZZAYO®2
STRIVE was a prospective, double-blind, randomized, dose-finding, phase 2 study of once-weekly intravenous REZZAYO® vs daily caspofungin for the treatment of candidemia and invasive candidiasis in patients age 18 and older. The trial was not powered to assess efficacy.1,2
Study design1,2
mITT N=183
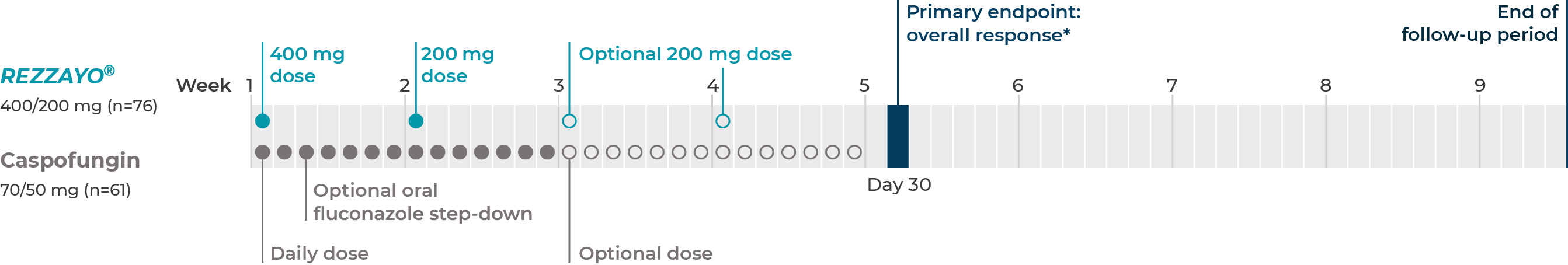
In the caspofungin group, optional oral fluconazole step-down therapy was permitted after ≥3 days of IV therapy if the patient met the criteria for cure and was preparing for discharge. Patients in the REZZAYO® group who were switched to step-down therapy continued to receive intravenous REZZAYO® once a week and daily oral placebo to maintain masking.2
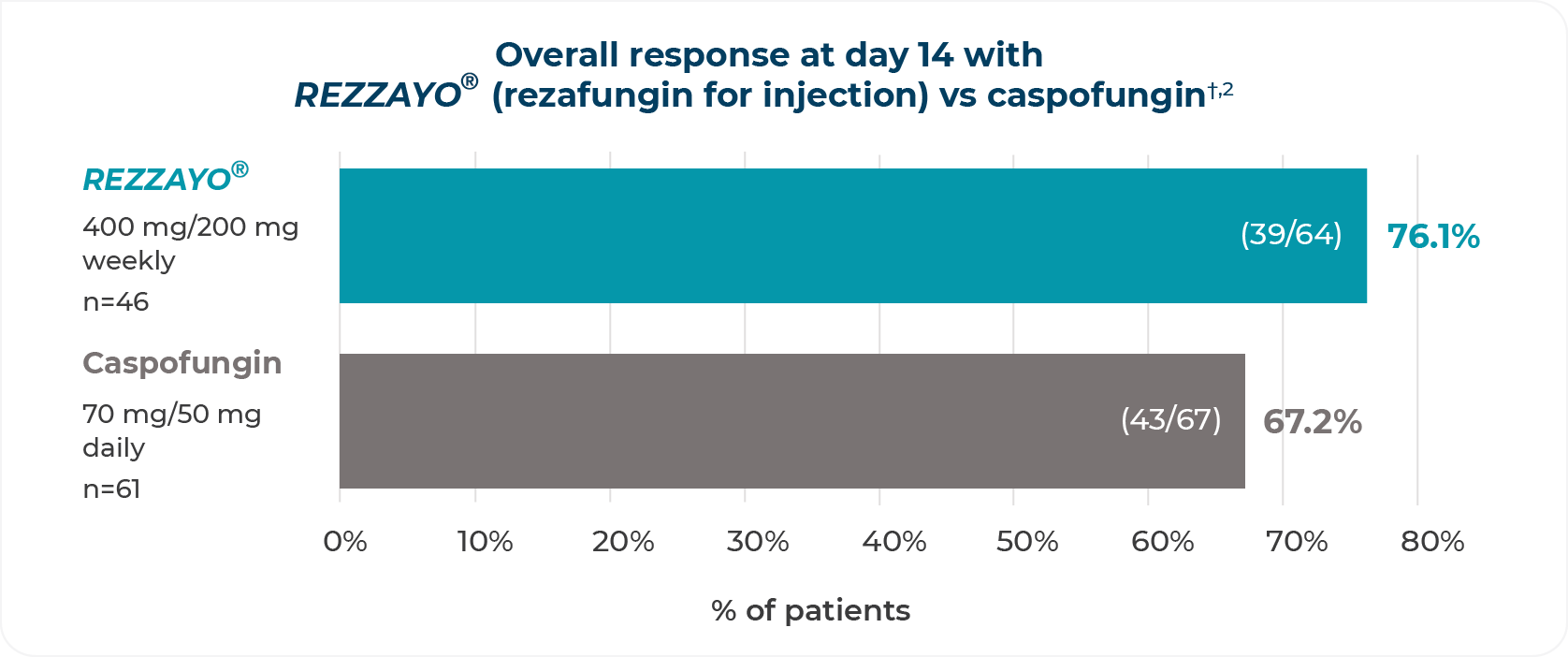
mITT=modified intent-to-treat.
*Overall response defined as overall cure (resolution of clinical signs of candidemia/invasive candidiasis) plus mycological eradication/presumed eradication.2
PRIMARY ENDPOINT: overall response at day 142
Similar results in secondary efficacy outcomes between REZZAYO® and caspofungin groups†,2
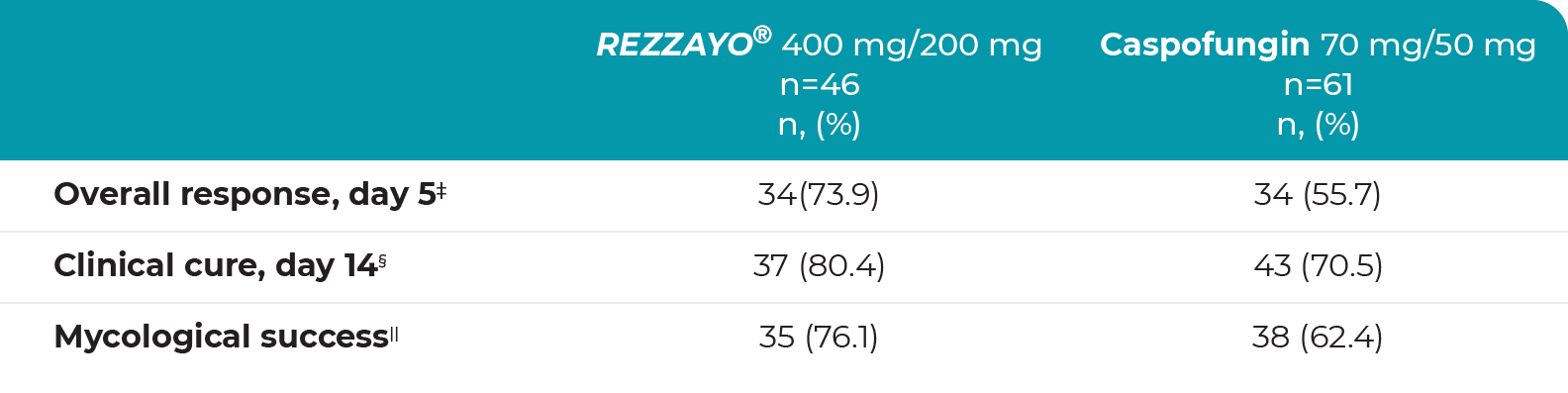
†The trial was not powered for inferential analysis.
‡Patients with mycological success (eradication/presumed eradication) and resolution of attributable systemic signs of candidemia/IC.
§Investigator’s assessment of clinical response based on resolution of attributable systemic signs and symptoms of candidemia/IC, no new systemic signs or symptoms attributable to candidemia/IC, no new systemic antifungal therapy to treat candidemia/IC, and the subject is alive.
||Negative blood culture or culture from a normally sterile site and no change in antifungal therapy for the treatment of candidemia and/or IC. For IC patients, if the normally sterile baseline site of Candida infection is not accessible, the patient is presumed to have an eradication if the clinical outcome is a cure.
IC=invasive candidiasis.
ReSTORE: rapid time to negative blood culture similar to caspofungin1,3
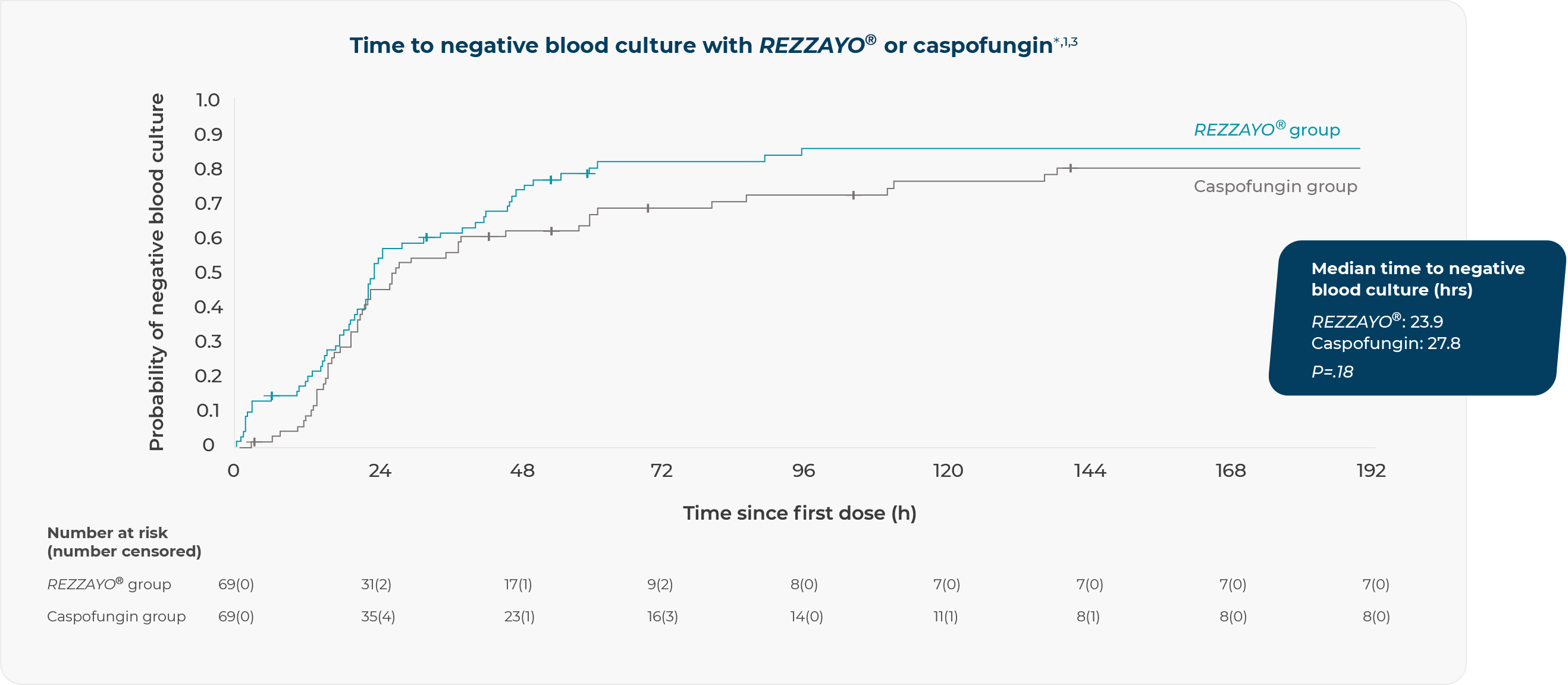
*Time to first negative blood culture (for patients enrolled with a positive blood culture) was a prespecified exploratory outcome of the ReSTORE phase 3 clinical trial and was measured (in hours) from the first dose of study drug to the first negative blood culture without subsequent positive culture.3
Fungicidal activity against the most common Candida species
ReSTORE: day 14 global cure and mycological eradication by Candida species in the mITT population3,5
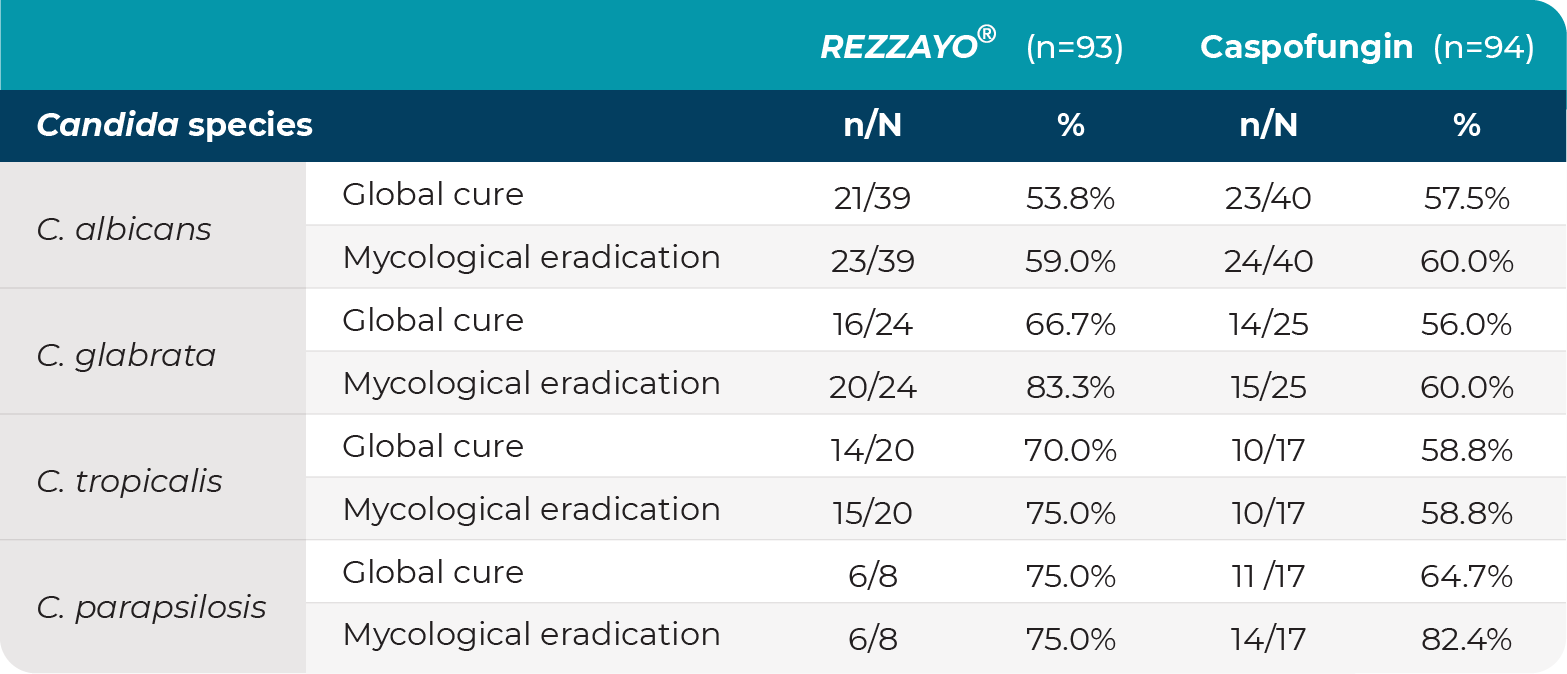
mITT=modified intent-to-treat.
 US
US BR
BR DE
DE ES
ES FR
FR IT
IT UK
UK
