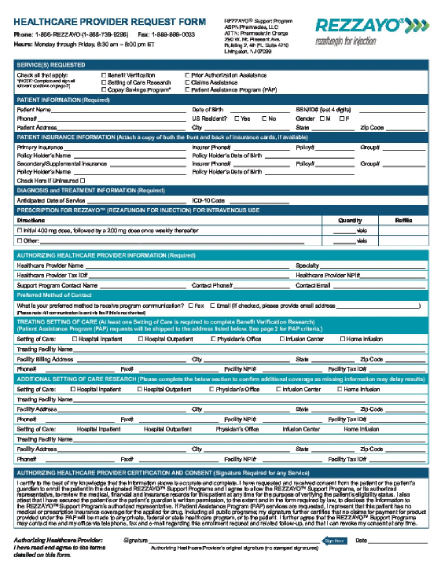
Support programs for REZZAYO®
Support, savings, and resources for patients
REZZAYO® offers a new option for continuity of echinocandin treatment in the outpatient setting.
Help appropriate patients start and stay on REZZAYO® with dedicated patient support programs.
Once-weekly REZZAYO®: Support program for patients
![]()
Benefits verification
![]()
Prior authorization (PA)
![]()
Claims appeal process
![]()
Copay assistance
![]()
Patient Assistance Program (PAP)
REZZAYO® coverage and process support
![]()
Benefits verification
- Obtain patient-specific insurance coverage for REZZAYO®
- Identify the patient’s cost-share responsibility (copay, coinsurance, deductible amounts)
- Verify PA requirements, if any
Prior authorization (PA) and claims appeal support
- Obtain PA requirements when requested by the payer
- Receive copies of payer-specific forms and a sample letter of medical necessity, if needed
- Stay informed throughout the entire process with updates on the status and outcome of the PA or appeal
REZZAYO® financial support programs
![]()
Copay assistance
- Copay support for eligible patients with private commercial insurance*
- Up to $400 per 200 mg vial
- Up to $800 for 400 mg loading dose
- No out-of-pocket minimum
- Patients must be 18 years of age or older and a US resident
- Patients must be treated in an outpatient setting of care
- There is no income requirement to qualify for copay assistance
![]()
Patient Assistance Program (PAP)
- Patients must meet household income requirements
- Both inpatients and outpatients may be eligible for the PAP
- Patients must be 18 years of age or older and a US resident
Patients must have a valid prescription for an FDA-approved indication.
*A patient will not qualify if they have a prescription drug benefit through a government program (i.e., Medicaid, Medicare, Medicare Part D, Medigap, CHAMPUS, DOD, VA, TRICARE, or any state patient or pharmaceutical assistance program).
As a condition precedent of the copayment or coinsurance support provided under this program, e.g., copay or coinsurance amounts paid to administering providers or participating patients, both are obligated to inform insurance companies and third-party payers of any benefits they receive and the value of this program, as required by contract or otherwise.
Void where prohibited by law, taxed, or restricted. Additional terms and conditions may apply. Patients enrolled in the REZZAYO® PAP are not eligible. Melinta Therapeutics, LLC may determine eligibility, monitor participation, and modify or discontinue any aspect of this program at any time.

Contact us for information and support:
| Phone |
1-866-REZZAYO (1-866-739-9296) |
| Hours |
Monday-Friday, 8:30 AM to 8:00 PM ET |
| Fax |
1-888-898-0033 |
| REZZAYO@Asembia.com |
Melinta Therapeutics, LLC does not guarantee that coverage or payment will occur for any particular claim.
Materials provided through the REZZAYO Support Programs are for informational purposes only. This information does not guarantee coverage or payment. Codes, coverage, and payment may vary from setting to setting, and from insurer to insurer.
The provider submitting a claim is solely responsible for the accuracy of the codes submitted and for compliance with all coverage and reimbursement policies.
Decisions to prescribe REZZAYO are made by providers working with their patients. The REZZAYO Support Programs provide information about REZZAYO and assistance in understanding its coverage and reimbursement. Patients who are not insured by a federal healthcare program and who meet certain other criteria may be eligible for financial assistance with their cost-sharing obligations. More information is available through the REZZAYO Support Programs hotline.
Melinta Therapeutics, LLC does not guarantee and assumes no responsibility for the quality, availability, or scope of the REZZAYO Support Programs services. Melinta Therapeutics, LLC reserves the right to rescind, revoke, or amend this offer at any time without notice.
 US
US BR
BR DE
DE ES
ES FR
FR IT
IT UK
UK